Pka Vinyl Hydrogen

Pka data compiled by r.
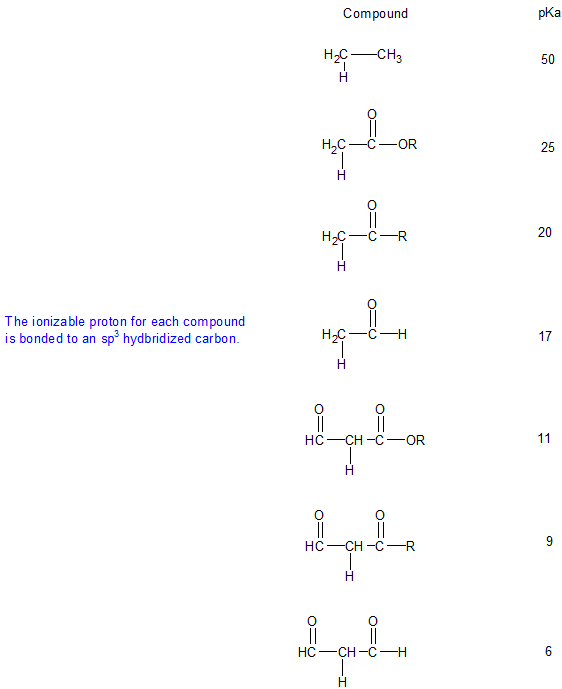
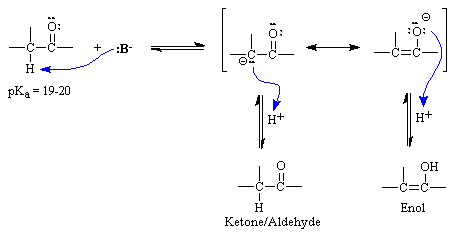
Pka vinyl hydrogen. Alpha proton of ketone aldehyde pka 20 11. Alcohol pka 16 18 8. Let s compare pk a of the common systems. Base methane and hydrogens on sp3carbons cyclopropane h.
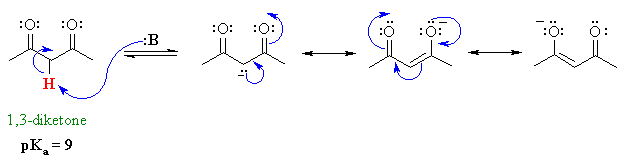
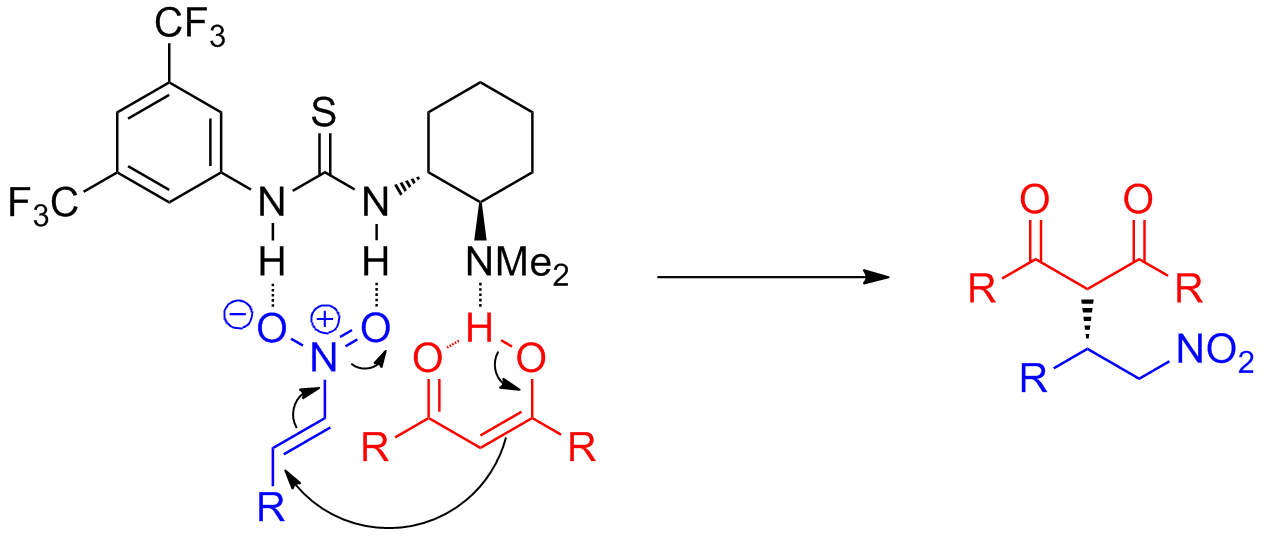
The difference between the 3 systems is in the nature of the group attached to the common carbonyl. Alpha proton of ester pka 25 12. Weakest acid on this table. Amide pka 18 10.
In this reaction accn is a radical initiator and an aliphatic thiol transfers radical character to the silylhydride. The aldehyde has a hydrogen the ketone an alkyl group and the ester an alkoxy group. In the laboratory silyl hydrides are used as reducing agent for example pmhs in one study triethylsilane is used in the conversion of a phenyl azide to an aniline. More vinyl than sp3 hybrid see below vinyl hydrogens.
Water pka 15 7 9. Williams page 1 pka values index inorganic 2 phenazine 24 phosphates 3 pyridine 25 carboxylic acids 4 8 pyrazine 26 aliphatic 4 8 aromatic 7 8 quinoline 27 phenols 9 quinazoline 27 alcohols and oxygen acids 10 11 quinoxaline 27 amino acids 12 special nitrogen compounds 28 peptides 13 hydroxylamines 28. Amine pka 38 40 14. Terminal alkyne pka 25 13.
The triethylsilyl free radical then reacts with the azide with expulsion of nitrogen to a. Aldehyde pk a 17 ketone pk a 19 and an ester pk a 25 and try to justify the trend. Hydrogen peroxide is a chemical compound with the formula h 2 o 2 in its pure form it is a very pale blue liquid slightly more viscous than water hydrogen peroxide is the simplest peroxide a compound with an oxygen oxygen single bond it is used as an oxidizer bleaching agent and antiseptic concentrated hydrogen peroxide or high test peroxide is a reactive oxygen species and has.